Postmarket Medical Device Safety Surveillance
This project aims to develop data extraction and processing methods to support real-world evidence studies for medical devices using electronic health record (EHR) data. Certain medical devices are approved based on limited clinical evidence, necessitating post-marketing evaluations to monitor the safety and effectiveness of the devices in specific patient populations. Real-world data generated during clinical care provides the opportunity to identify safety findings not detected in smaller pre-market trials, monitor known safety concerns, and assess long-term outcomes. For several medical devices with potential safety issues, we are using EHR data to develop methods to support post-marketing evaluations and assess specific safety concerns.
Safety Events Associated With Endovascular Aneurysm Repair Devices
One such study has assessed safety events related to endovascular aneurysm repair (EVAR) devices. This project applies advanced informatics and statistical pipelines to assess the association of EVAR devices with rare and long-term safety outcomes in unstructured medical record notes. Find more information on the study below:
Read the accompanying editorial and viewpoint.
Additional information on EVAR device safety concerns can be found on the FDA website.
Intervertebral Body Fusion Devices
The Center has been involved in two studies researching the manufacturing processes of different intervertebral body fusion devices (IVBD), and how these manufacturing processes contribute to patient safety, device longevity, and re-operation risks. Each project seeks to generate real-world evidence to determine trade-offs in safety and efficacy related to novel IVBDs, with the goal of ascertaining the value of manufacturing advancements in IVBDs, and helping to guide regulatory standards and decisions for these novel device types.
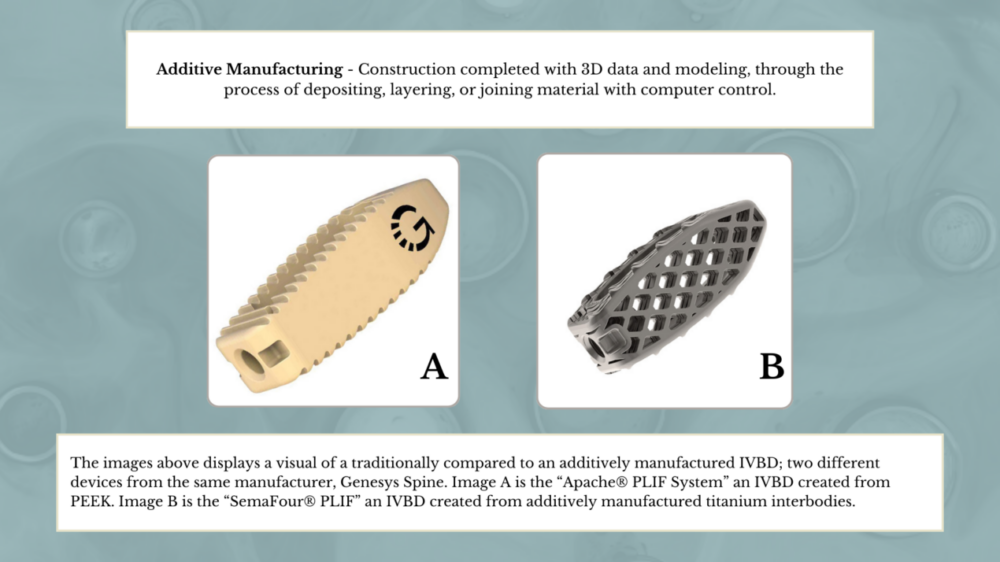
The first study examines the difference between traditionally and additively manufactured (AM) IVBDs. Traditionally manufactured devices are typically made from polyetheretherketone (PEEK) or titanium, while new additively manufactured devices are powdered titanium alloy and 3D printed. Initial benefits of AM-IVBDs are larger contact areas, enhanced mechanical properties, better biocompatibility, and personalized construction for better anatomic fit for patients. However, bench testing shows that these types of devices tend to be more brittle and have poorer fatigue properties than their traditionally manufactured counterparts. The study is researching the rate of adverse events, including risk of re-operation, between these different IVBD types.
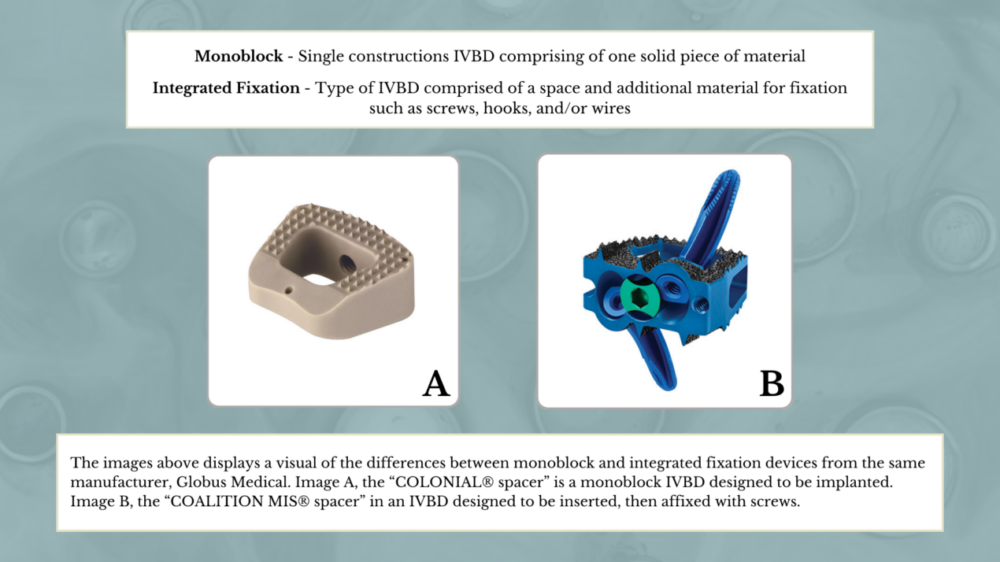
The second study also compares two different classes of IVBDs: monoblock devices and integrated fixation devices. Each class of IVBDs is affixed in a different manner and provides different benefits. Devices with integrated fixation rose in prominence due to their ability to provide increased stability, reduce clinical complications, and increase fusion rates. However, a cohort study where spinal fusion was performed with both classes of IVBDs showed monoblock devices to be more advantageous. These stand-alone devices were associated with less blood loss, improved patient satisfaction, and decreased surgical time. The study examines the rate of specific types of adverse events associated with these two classes of IVBDs.