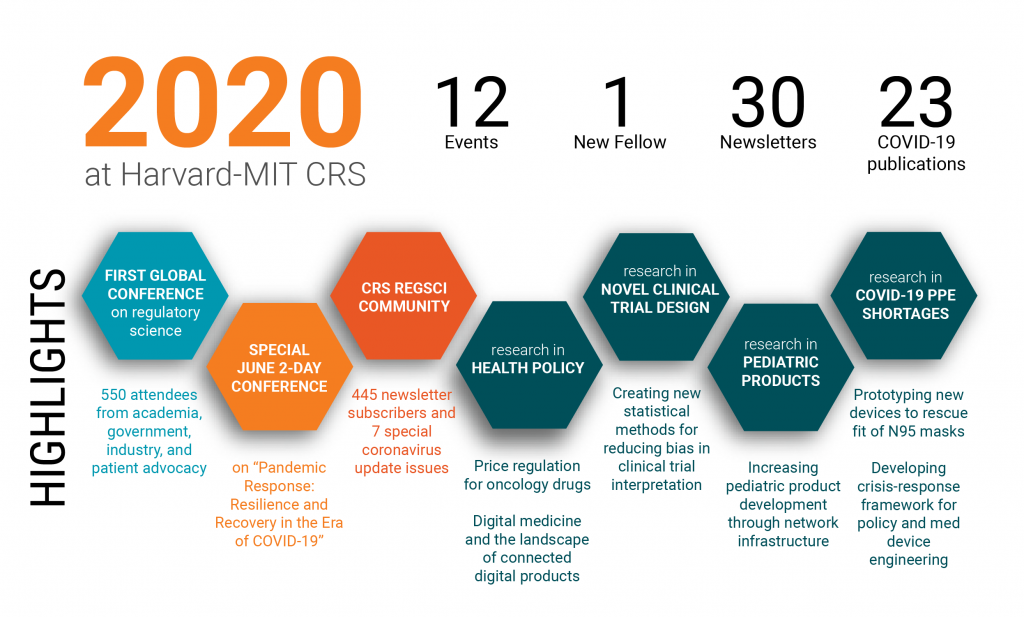
Year in Review: 2020 at Harvard-MIT CRS
We hope that all of you in our regulatory science community, and your families, are staying safe and healthy. We recognize that 2020 has been a challenging year for all of us as we have learned to cope, in large and small ways, with the COVID-19 pandemic.
In spite of the challenges, we have accomplished a lot! In addition to addressing PPE shortages and issues of coordination around the pandemic, we hosted our first Global Conference on Regulatory Science and continued to publish research in digital medicine, novel clinical trial designs, health policy, real world evidence, pediatric drug and device development, and biomarkers and surrogate markers in drug trials. Thanks for being with us on this journey, we hope that we can continue to support each other and that we will emerge in 2021 as a stronger and more united community in regulatory science and beyond.